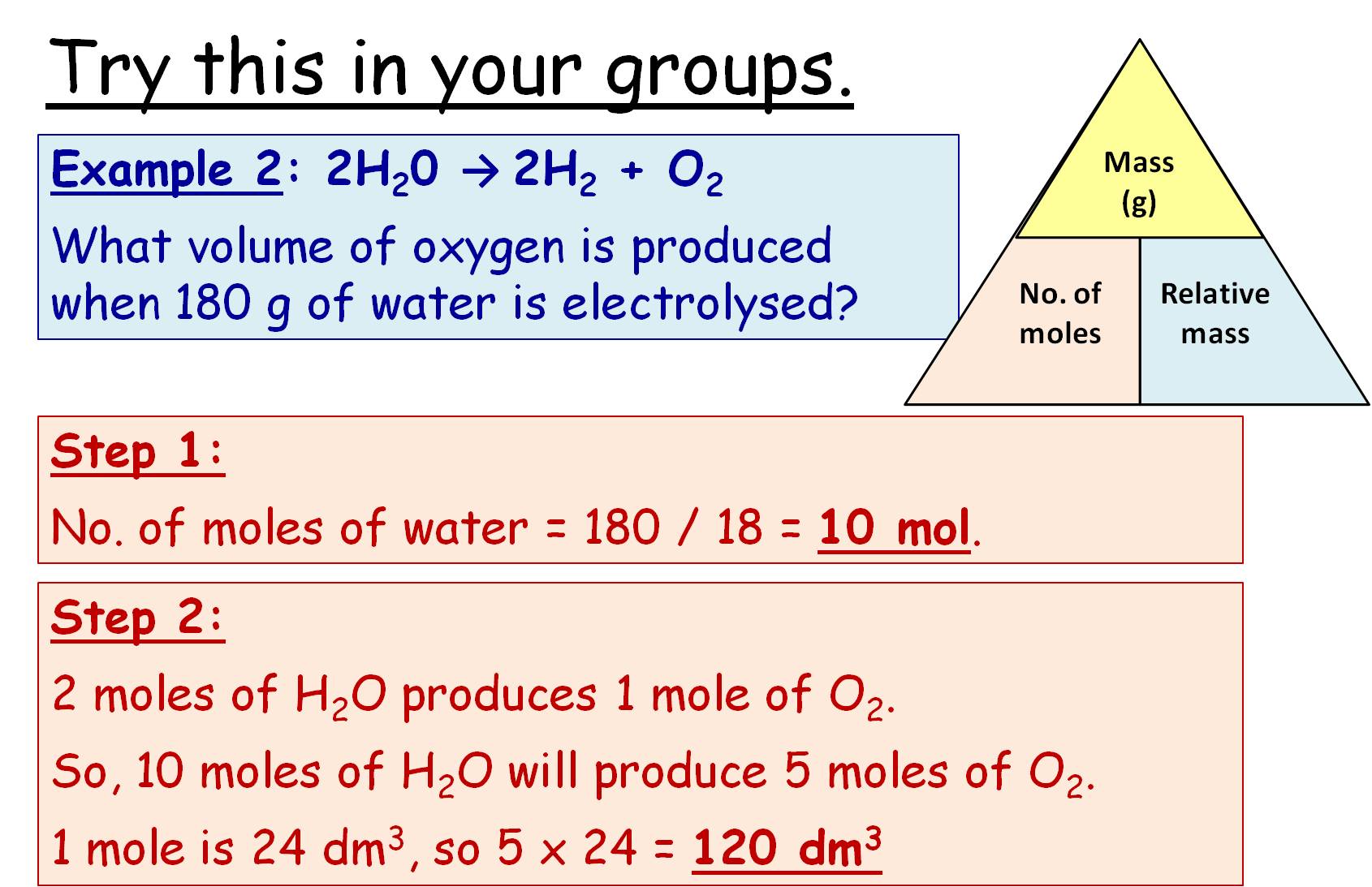
How Do You Calculate Volume Of Gas . — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume of a. easily calculate the pressure, volume, temperature or quantity in moles of a gas using this combined gas law calculator. with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. — the volume of a gas may be calculated from its number of moles using: Volume of gas = moles × v m. — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. — this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or. — do you want to know how to calculate the volume of gases?
from www.tes.com
with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. — this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or. easily calculate the pressure, volume, temperature or quantity in moles of a gas using this combined gas law calculator. — do you want to know how to calculate the volume of gases? — the volume of a gas may be calculated from its number of moles using: — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume of a. — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. Volume of gas = moles × v m.
Molar Volume of Gases GCSE Lesson (SC14e) TRIPLE Teaching Resources
How Do You Calculate Volume Of Gas — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume of a. — this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or. with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. Volume of gas = moles × v m. — the volume of a gas may be calculated from its number of moles using: — do you want to know how to calculate the volume of gases? easily calculate the pressure, volume, temperature or quantity in moles of a gas using this combined gas law calculator.
From www.youtube.com
Gas volumes (AQA GCSE Chemistry) YouTube How Do You Calculate Volume Of Gas with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. — do you want to know how to. How Do You Calculate Volume Of Gas.
From www.youtube.com
Calculando o volume de um gás ideal YouTube How Do You Calculate Volume Of Gas — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume of a. — the volume of a gas may be calculated from its number of moles using: — this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or. easily. How Do You Calculate Volume Of Gas.
From abbieyouthclayton.blogspot.com
How to Calculate Volume of Gas How Do You Calculate Volume Of Gas Volume of gas = moles × v m. — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume of a. with the ideal gas. How Do You Calculate Volume Of Gas.
From www.youtube.com
11.8 The Ideal Gas Law Pressure, Volume, Temperature, & Moles YouTube How Do You Calculate Volume Of Gas Volume of gas = moles × v m. — this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or. — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume of a. with the ideal gas law, we can use the. How Do You Calculate Volume Of Gas.
From www.slideserve.com
PPT Moles and gas volumes PowerPoint Presentation, free download ID How Do You Calculate Volume Of Gas with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. easily calculate the pressure, volume, temperature or quantity. How Do You Calculate Volume Of Gas.
From peacecommission.kdsg.gov.ng
Molar Gas Volume Avogadro's Law Moles And Mass Calculations Gcse How Do You Calculate Volume Of Gas — do you want to know how to calculate the volume of gases? with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles. How Do You Calculate Volume Of Gas.
From www.youtube.com
Volume of compressed gas in cylinder YouTube How Do You Calculate Volume Of Gas — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume of a. easily calculate the pressure, volume, temperature or quantity in moles of a. How Do You Calculate Volume Of Gas.
From www.thetechedvocate.org
How to calculate volume of gas The Tech Edvocate How Do You Calculate Volume Of Gas with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. — do you want to know how to calculate the volume of gases? — the volume of a gas may be calculated from its number of moles using: Volume of gas = moles ×. How Do You Calculate Volume Of Gas.
From www.grc.nasa.gov
Specific Volume How Do You Calculate Volume Of Gas — the volume of a gas may be calculated from its number of moles using: — this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or. easily calculate the pressure, volume, temperature or quantity in moles of a gas using this combined gas law calculator. —. How Do You Calculate Volume Of Gas.
From www.tes.com
Gas Volumes and Gas Calculations Edexcel 91 Separate (Triple) Science How Do You Calculate Volume Of Gas — this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or. easily calculate the pressure, volume, temperature or quantity in moles of a gas using this combined gas law calculator. — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume. How Do You Calculate Volume Of Gas.
From en.ppt-online.org
The ideal gas equation online presentation How Do You Calculate Volume Of Gas — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. Volume of gas = moles × v m. . How Do You Calculate Volume Of Gas.
From www.showme.com
Volume gas to Moles Science, Chemistry, Stoichiometry ShowMe How Do You Calculate Volume Of Gas — do you want to know how to calculate the volume of gases? easily calculate the pressure, volume, temperature or quantity in moles of a gas using this combined gas law calculator. — the volume of a gas may be calculated from its number of moles using: with the ideal gas law, we can use the. How Do You Calculate Volume Of Gas.
From www.youtube.com
Molar Mass of a Gas at STP Equations & Formulas, Chemistry Practice How Do You Calculate Volume Of Gas easily calculate the pressure, volume, temperature or quantity in moles of a gas using this combined gas law calculator. — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. — charles' law (sometimes referred to as the law of volumes) describes. How Do You Calculate Volume Of Gas.
From mmerevise.co.uk
The Ideal Gas Equation MME How Do You Calculate Volume Of Gas Volume of gas = moles × v m. easily calculate the pressure, volume, temperature or quantity in moles of a gas using this combined gas law calculator. — this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or. — charles' law (sometimes referred to as the law. How Do You Calculate Volume Of Gas.
From www.youtube.com
Calculate the volume of 1 mole of an ideal gas at STP. YouTube How Do You Calculate Volume Of Gas with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. Volume of gas = moles × v m. — calculate any variable in the equation for the ideal gas law pv = nrt, where pressure times volume equals moles times the ideal gas constant. . How Do You Calculate Volume Of Gas.
From www.savemyexams.co.uk
Calculate Volumes of Gases (1.5.10) Edexcel IGCSE Chemistry Revision How Do You Calculate Volume Of Gas — do you want to know how to calculate the volume of gases? — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume of a. — the volume of a gas may be calculated from its number of moles using: easily calculate the pressure, volume, temperature or quantity in. How Do You Calculate Volume Of Gas.
From vernonrybolt1967.blogspot.com
Vernon Rybolt How To Calculate Volume Of Gas From Moles How Do You Calculate Volume Of Gas easily calculate the pressure, volume, temperature or quantity in moles of a gas using this combined gas law calculator. with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. — charles' law (sometimes referred to as the law of volumes) describes the relationship between. How Do You Calculate Volume Of Gas.
From taylorwindam1952.blogspot.com
Taylor Windam How Do You Measure Volume Of A Gas How Do You Calculate Volume Of Gas — charles' law (sometimes referred to as the law of volumes) describes the relationship between the volume of a. — this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or. — do you want to know how to calculate the volume of gases? with the ideal. How Do You Calculate Volume Of Gas.